Would you like to make this site your homepage? It's fast and easy...
Yes, Please make this my home page!
HOME~~~PREV~~~NEXT
? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ?
? ?
? WHAT ELEMENTS OR COMPOUNDS ?
? ?
? ACTUALLY HOLD OR CARRY ?
? ?
? THE ELECTRONS AND HYDROGEN ATOMS ?
? ?
? IN THE TRANSFER SEQUENCES ?
? ?
? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ?
~~~ BIOLOGICALLY IMPORTANT CARRIERS OF REDUCING EQUIVALENTS ~~~
METALS:
Fe, Fe2S2, Fe4S4, Cu, Mn, Mo
HYDROXYL GROUPS:
alcohols, sugars, hydroxycarboxylic acids,
enediols, phenols, polyphenols, quinols
SULFHYDRYL GROUPS:
glutathione, protein thiols, lipoic acid
IMINES:
pyridiniums, flavins, pyrroles,
phenazines, biopterin, folates
~~~ TRANSITION METALS ~~~
So named according to their position in the periodic table,
transition metals produce cations of various valences.
Because fairly low voltage changes are associated with
these valence changes, this characteristic is useful
to pick up and hold electrons under the relatively
mild reaction conditions associated with life.
Iron, copper, and manganese activate numerous enzymes for
purposes of oxidatizing substrates, holding electrons,
moving electrons, or reducing substrates.
ELEMENT: METAL: VALENCES:
iron Fe Fe++ , Fe+++
Fe++++ , Fe++++++
copper Cu Cu+ , Cu++ , Cu+++
manganese Mn Mn++ , Mn+++ , Mn++++
Mn++++++ , Mn+++++++
~~~ FUNCTIONS OF COPPER ~~~
CYTOCHROME A
utilizes oxygen in mitochondria
SUPEROXIDE DISMUTASE
removes -OO* and produces H2O2
CERULOPLASMIN
carries copper to tissues
eliminates superoxide from the blood
recycles enediols and paraquinones
AMINE OXIDASES
remove: ptomaines, polyamines, histamine,
neurotransmitters, vasopressors
LYSYL OXIDASE
converts lysine to aldehyde to crosslink elastin
CHOLESTEROL OXIDATION
oxidative elimination of cholesterol
~~~ ORGANIC SULFUR OXIDES ~~~
Due to its size and outer orbital characteristics,
sulfur is highly versatile in its covalent bonding
capabilities with other sulfur molecules and with oxygen.
NAMES: elemental sulfur FORMULAE: S8
thiol RSH
alkyl disulfide RSSR'
disulfide monoxide RSSOR'
disulfide dioxide RSSO2R'
disulfide trioxide RSOSO2R'
disulfide tetraoxide RSO2SO2R'
dialkyl sulfide RSR'
dialkyl sulfoxide RSOR'
dialkyl sulfone RSO2R'
sulfenic acid RSOH
sulfinic acid RSO2H
sulfonic acid RSO3H
sulfonamide RSO2NH2
alcohol sulfate ester ROSO3H
~~~ THIOLS / SULFHYDRYL COMPOUNDS ~~~
Thiols have the general formula RSH.
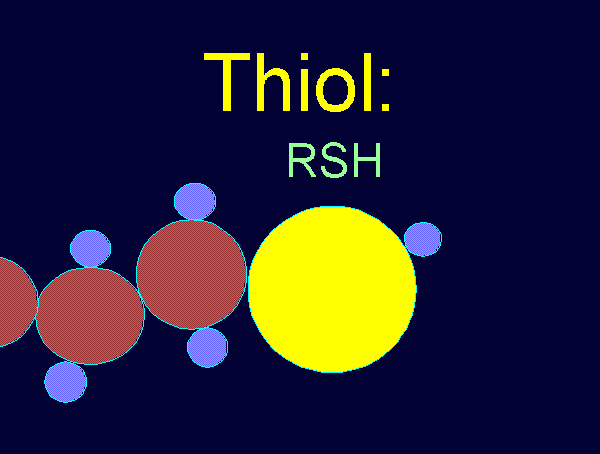 Thiols are readily oxidized by a variety of oxidants
to produce the relatively stable thiyl radical (RS*).
RSH + [O] ---> RS* + H[O]*
Thiols are readily oxidized by a variety of oxidants
to produce the relatively stable thiyl radical (RS*).
RSH + [O] ---> RS* + H[O]*
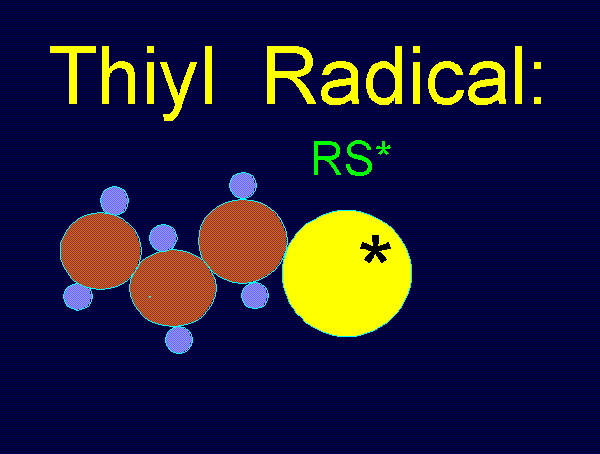 Thiyl radicals readily couple to produce disulfides (RSSR').
RS* + R'S* ---> RSSR'
Thiyl radicals readily couple to produce disulfides (RSSR').
RS* + R'S* ---> RSSR'
 These actions make thiols such as glutathione,
thioredoxin, and other peptides containing the
amino acid cysteine handy carriers of hydrogen atoms.
~~~ THIOL OXIDATION BY OXYRADICALS ~~~
Hydroxyl Radical:
HO* + RSH ---> HOH + RS*
Hydroperoxyl Radical:
HOO* + RSH ---> HOOH + RS*
Alkoxyl Radical:
RO* + RSH ---> ROH + RS*
Alkylperoxyl Radical:
ROO* + RSH ---> ROOH + RS*
~~~ THIOL OXIDATION BY TRANSITION METALS ~~~
RSH + Fe+++ ---> RS* + Fe++ + H+
~~~ THIOL OXIDATION BY QUINONES ~~~
RSH + Q ---> RS* + HQ*
~~~ THIOLS AS WEAK ACIDS ~~~
RSH + B- ---> RS- + HB
- Thiols can deprotonate at pH = 8.
- This produces the "thiolate anion" (RS-).
- Thiolate can function as a nucleophile.
- Thiolate is more sensitive to oxidation
than the corresponding thiol was.
~~~ THIOL ALDEHYDE ADDITIONS ~~~
O OH
R-S-H + C-R' <---> R-S-C-R'
H H
Thiols and aldehydes react by nucleophilic
addition to produce "thiohemiacetals".
By this mechanism, many enzymes having
thiol(s) as part of the active center are
reversibly inhibited by aldehydes.
Also by this mechanism, certain aldehydes
are detoxified by thiols.
~~~ THIOL / DISULFIDE EXCHANGE REACTIONS ~~~
Such reactions are catalized by thiolate anion and are
therefore facilitated by mildly alkaline conditions.
Thiols acting as nucleophiles can undergo
exchanges with disulfides:
RSH + R'SSR" ---> R'SSR + HSR"
Hydrogen sulfide similarly adds to disulfides
displacing a thiol:
HSH + R'SSR" ---> R'SSH + HSR"
Disulfides can exchange bound thyil groups:
RSSR' + R"SSR''' ---> RSSR''' + R"SSR'
~~~ THIOL OXIDATION TO DISULFIDE
BY NON-FREE-RADICAL MECHANISMS ~~~
RSH + R'SH + [O] ---> RSSR' + H[O]H
Examples below show the ubiquitous thiol glutathione
(GSH) being oxidized by:
sulfur: S8 + 16 GSH ---> 8 H2S + 8 GSSG
disulfide: RSSR + 2 GSH ---> 2 RSH + GSSG
sulfoxide: RSOR + 2 GSH ---> R-S-R + GSSG + H2O
disulfide monoxide:
RSSOR + 4 GSH ---> 2 RSH + 2 GSSG + H2O
diamide: D + 2 GSH ---> DH2 + GSSG
enzyme: Enz + 2 GSH ---> EnzH2 + GSSG
~~~ DISULFIDE-MONOXIDE AND THIOL REACTIONS ~~~
Disulfide-monoxides (RSOSR') first exchange
with thiols and then oxidize them.
RSOSR' + R"SH ---> RSOH + R'SSR"
RSOH + R"SH ---> HOH + RSSR"
The end result is two oxidized disulfides,
capable of absorbing four hydrogen atoms.
Allicin is a disulfide monoxide, a mild
oxidant, and an antibacterial agent.
H H O H H
C==C--C--S--S--C--C==C
H H H H H H
~~~ THIOL OXIDATION BY DIAMIDE ~~~
R'SH + R"SH + D ---> R'SSR" + DH2
- Diamide is a specific oxidant for thiols.
- It acts most rapidly upon low molecular
weight thiols such as glutathione.
- It works by a combined nucleophilic addition
& displacement mechanism, which cleanly
converts thiols to disulfides.
- It penetrates all compartments rapidly
and is effective both in vivo & in vitro.
- These features make diamide a useful probe
to study thiol to disulfide conversions.
------------------------------------------------
H3C O O CH3
\ || || /
N---C---N===N---C---N
/ \
H3C CH3
---------------------------------------------
R'--SH R'--S
---> \ H
--N==N-- --N--N--
---------------------------------------------
HS--R" R'--S--S--R"
R'--S
\ H ---> H H
--N--N-- --N--N--
---------------------------------------------
~~~ THIOLS AS CARRIERS OF ACYL GROUPS ~~~
R--S--C--R'
||
O
~~~ BIOLOGICAL THIOLS ~~~
NOTE: These naturally occuring thiols participate
in numerous physiologic functions and are readily
and reversibly oxidizable to disulfides.
"Co-Enzyme A" "Thioctic (Lipoic) Acid"
"Cysteine" "Glutathione" "Protein Thiols"
---------------------------------------------------------
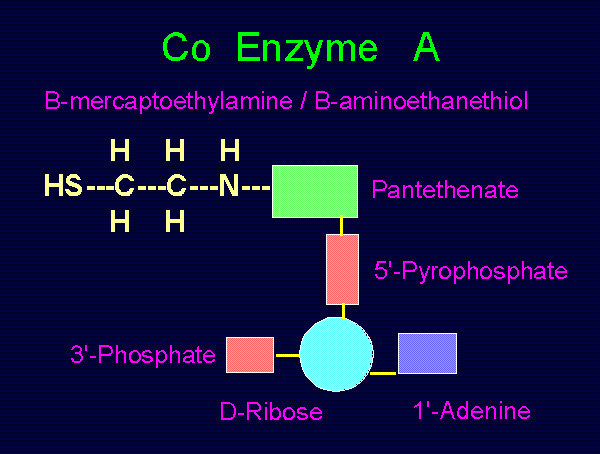
CO-ENZYME A:
beta-amino-ethane-thiol 1'adenine
H H H /
HS--C--C--N--pantethine--5'pyrophosphate--ribose
H H \
3'phosphate
These actions make thiols such as glutathione,
thioredoxin, and other peptides containing the
amino acid cysteine handy carriers of hydrogen atoms.
~~~ THIOL OXIDATION BY OXYRADICALS ~~~
Hydroxyl Radical:
HO* + RSH ---> HOH + RS*
Hydroperoxyl Radical:
HOO* + RSH ---> HOOH + RS*
Alkoxyl Radical:
RO* + RSH ---> ROH + RS*
Alkylperoxyl Radical:
ROO* + RSH ---> ROOH + RS*
~~~ THIOL OXIDATION BY TRANSITION METALS ~~~
RSH + Fe+++ ---> RS* + Fe++ + H+
~~~ THIOL OXIDATION BY QUINONES ~~~
RSH + Q ---> RS* + HQ*
~~~ THIOLS AS WEAK ACIDS ~~~
RSH + B- ---> RS- + HB
- Thiols can deprotonate at pH = 8.
- This produces the "thiolate anion" (RS-).
- Thiolate can function as a nucleophile.
- Thiolate is more sensitive to oxidation
than the corresponding thiol was.
~~~ THIOL ALDEHYDE ADDITIONS ~~~
O OH
R-S-H + C-R' <---> R-S-C-R'
H H
Thiols and aldehydes react by nucleophilic
addition to produce "thiohemiacetals".
By this mechanism, many enzymes having
thiol(s) as part of the active center are
reversibly inhibited by aldehydes.
Also by this mechanism, certain aldehydes
are detoxified by thiols.
~~~ THIOL / DISULFIDE EXCHANGE REACTIONS ~~~
Such reactions are catalized by thiolate anion and are
therefore facilitated by mildly alkaline conditions.
Thiols acting as nucleophiles can undergo
exchanges with disulfides:
RSH + R'SSR" ---> R'SSR + HSR"
Hydrogen sulfide similarly adds to disulfides
displacing a thiol:
HSH + R'SSR" ---> R'SSH + HSR"
Disulfides can exchange bound thyil groups:
RSSR' + R"SSR''' ---> RSSR''' + R"SSR'
~~~ THIOL OXIDATION TO DISULFIDE
BY NON-FREE-RADICAL MECHANISMS ~~~
RSH + R'SH + [O] ---> RSSR' + H[O]H
Examples below show the ubiquitous thiol glutathione
(GSH) being oxidized by:
sulfur: S8 + 16 GSH ---> 8 H2S + 8 GSSG
disulfide: RSSR + 2 GSH ---> 2 RSH + GSSG
sulfoxide: RSOR + 2 GSH ---> R-S-R + GSSG + H2O
disulfide monoxide:
RSSOR + 4 GSH ---> 2 RSH + 2 GSSG + H2O
diamide: D + 2 GSH ---> DH2 + GSSG
enzyme: Enz + 2 GSH ---> EnzH2 + GSSG
~~~ DISULFIDE-MONOXIDE AND THIOL REACTIONS ~~~
Disulfide-monoxides (RSOSR') first exchange
with thiols and then oxidize them.
RSOSR' + R"SH ---> RSOH + R'SSR"
RSOH + R"SH ---> HOH + RSSR"
The end result is two oxidized disulfides,
capable of absorbing four hydrogen atoms.
Allicin is a disulfide monoxide, a mild
oxidant, and an antibacterial agent.
H H O H H
C==C--C--S--S--C--C==C
H H H H H H
~~~ THIOL OXIDATION BY DIAMIDE ~~~
R'SH + R"SH + D ---> R'SSR" + DH2
- Diamide is a specific oxidant for thiols.
- It acts most rapidly upon low molecular
weight thiols such as glutathione.
- It works by a combined nucleophilic addition
& displacement mechanism, which cleanly
converts thiols to disulfides.
- It penetrates all compartments rapidly
and is effective both in vivo & in vitro.
- These features make diamide a useful probe
to study thiol to disulfide conversions.
------------------------------------------------
H3C O O CH3
\ || || /
N---C---N===N---C---N
/ \
H3C CH3
---------------------------------------------
R'--SH R'--S
---> \ H
--N==N-- --N--N--
---------------------------------------------
HS--R" R'--S--S--R"
R'--S
\ H ---> H H
--N--N-- --N--N--
---------------------------------------------
~~~ THIOLS AS CARRIERS OF ACYL GROUPS ~~~
R--S--C--R'
||
O
~~~ BIOLOGICAL THIOLS ~~~
NOTE: These naturally occuring thiols participate
in numerous physiologic functions and are readily
and reversibly oxidizable to disulfides.
"Co-Enzyme A" "Thioctic (Lipoic) Acid"
"Cysteine" "Glutathione" "Protein Thiols"
---------------------------------------------------------
CO-ENZYME A:
beta-amino-ethane-thiol 1'adenine
H H H /
HS--C--C--N--pantethine--5'pyrophosphate--ribose
H H \
3'phosphate
 ---------------------------------------------------------
THIOCTIC (LIPOIC) ACID:
Dihydrothioctic Acid (reduced):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
| | |
SH SH OH
Thioctic Acid (oxidized):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
\ / |
S---S OH
---------------------------------------------------------
ANALOGUES OF CYSTEINE:
---------------------------------------------------------
SERINE: COOH
\ H oxygen as in alcohol
HC--C--OH
/ H ^
NH2
---------------------------------------------------------
CYSTEINE: COOH
\ H sulfur as in thiol
HC--C--SH
/ H ^
NH2
---------------------------------------------------------
SELENOCYSTEINE: COOH
\ H selenium as in selenol
HC--C--SeH
/ H ^
NH2
---------------------------------------------------------
GLUTATHIONE (GSH) :
(gamma-glutamylcysteinylglycine)
COOH O O O
\ H H || H || H ||
C--C--C--C--N--C--C--N--C--C
/ H H H | H H \
NH2 HCH OH
|
SH
GLUTATHIYL RADICAL (GS*) :
[tripeptide]--S*
GLUTATHIONE DISULFIDE (GSSG) :
[tripeptide]--S--S--[tripeptide]
~~~ PROTEIN THIOLS ~~~
These include redox active cysteine residues
in their amino acid sequences.
They function as hydrogen carriers, physiologic
triggers, redox buffers, and oxidoreductases.
Examples: - Thioredoxin
- Glutaredoxin
- Protein Disulfide Isomerase
- Ref-1
- Transcription Factors
- Growth Factors
- Metallothionein
---------------------------------------------------------
PROTEIN THIOLS:
(Note reduced condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| | |
SH SH SH
(Note oxidized condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| \ /
S--S--R S--S
---------------------------------------------------------
~~~ MECHANISM OF OXIDO-REDUCTASES ~~~
NOTE: These use the thiol / disulfide half reaction
as part of the reductant transfer mechanism.
1) Red-H2 + Enz-SS ---> Red + Enz-(SH)2
2) Enz-(SH)2 + Ox ---> Ox-H2 + Enz-SS
- cys - - cys - - cys -
| | |
H S SH S H
[Red] | [Ox] ---> [Red] [Ox] ---> [Red] | [Ox]
H S SH S H
| | |
- cys - - cys - - cys -
~~~ MECHANISM OF INACTIVATION BY MERCURY II ~~~
Enz-(SH)2 + Hg++ ---> Enz-SHgS + 2H+
- cys - - cys - | - cys -
| | | |
SH S | H S
+ Hg++ ---> Hg + 2H+ | [Red] Hg [Ox]
SH S | H S
| | | |
- cys - - cys - | - cys -
~~~ ENOLS MECHANISM OF DEHYDROGENATION ~~~
Enols are composed of one hydroxyl group (HO)
attached to the vinylic carbon of an olefin.
OH
|
-C==C-
When oxidized by the abstraction of the
hydroxyl hydrogen, an alkoxyl radical is produced.
It is stabilized by two point resonance
with the conjugated vinyl group.
O* O
| <---> | *
-C==C- -C--C-
If this structure is further conjugated,
then fairly stable radicals can be produced.
~~~ UNPROTECTED PHENOXYL RADICAL ~~~
O* O O O
| || || ||
C C C C
// \ / \ / \ / \
HC CH HC* CH HC CH HC *CH
| || | || || || || |
HC CH HC CH HC CH HC CH
\\ / \\ / \ * / \ //
C C C C
H H H H
Phenoxyl radicals are stabilized well by 4 point resonance.
However, they are subject to addition reactions at each
of the 4 exposed radical sites shown: O, C#2, C#4, C#6.
~~~ PROTECTED PHENOXYL RADICALS ~~~
NOTE: The attached groups protect the four sites
of resonance against addition reactions.
-------------------------------------------------------
butylatedhydroxytoluene HC--C--C(CH3)3
RO* + BHT-OH --> // \\
ROH + BHT-O* CH3--C C--O*
\ /
HC==C--C(CH3)3
-------------------------------------------------------
alpha-tocopherol CH3 CH3--C--C--CH3
RO* + E-OH --> \ // \\
ROH + E-O* [turpene]--C---O--C C--O*
| \ /
CH2-CH2--C==C--CH3
-------------------------------------------------------
ubiquinol H3CO--C--C--OCH3
RO* + CoQ-OH --> // \\
ROH + CoQ-O* HO--C C--O*
\ /
[turpene]--C==C--CH3
-------------------------------------------------------
~~~ ENEDIOLS MECHANISM OF DEHYDROGENATION ~~~
Enediols are subject to facile one and two step
hydrogen abstractions.
STEP 1: OH OH O* OH O OH
| | + [O] ---> | | <---> || |
--C==C-- --C==C-- -C--C--
*
STEP 2: O OH O O* O O
|| | + [O] ---> || | ---> || ||
--C--C-- --C--C-- --C--C--
* *
The first abstraction is more difficult, because the
strong covalent oxyhydrogen sigma bond must be broken
to generate an alkoxyl radical.
However the opportunity for resonance stabilization
offerred by the conjugated vinyl group energetically
facilitates the abstraction.
Resonance towards the second hydroxyl group facilitates
release of the second hydrogen atom, because all
electrons of the final product can be paired.
This is an example of free radical quenching by oxidation.
~~~ ENEDIOL EXAMPLES ~~~
-------------------------------------------------
ASCORBIC ACID HO OH
\ /
H C==C
H O / \
HC--C--CH C==O
O H \ /
H O
-------------------------------------------------
CATACHOLS/ HO OH
ORTHOHYDROQUINOLS \ /
C==C
/ \
R--C C--R
\\ //
R--C--C--R
~~~ PARAHYDROQUINOL MECHANISM OF DEHYDROGENATION ~~~
Parahydroquinol (QH2):
HC==CH HC==CH
/ \ / \
HO-C C-OH + [O] ---> *O-C C-OH + *[O]H
\\ // \\ //
HC--CH HC--CH
Semiquinone (*QH):
HC==CH <---> HC==CH <---> HC==CH <---> HC--CH
/ \ / \ / \ /* \\
*O-C C-OH O=C C-OH O=C *C-OH O=C C-OH
\\ // \* // \ / \ /
HC--CH HC--CH HC==CH HC==CH
Paraquinone (Q):
HC==CH HC==CH
/ \ / \
O=C *C-OH + [O] ---> O=C C=O + *[O]H
\ / \ /
HC==CH HC==CH
~~~ IMINES ~~~
Imines (also known as Schiff's bases) are compounds
containing the carbon to nitrogen double bond.
C==N
They can be produced by the reaction of a carbonyl
group and a primary amino group at slightly acid pH.
\ H \ /OH \
C=O + N-R <---> C <---> C=N-R + HOH
/ H / \N-R /
H
As indicated by the arrows,
this reaction is usually reversible.
Imines can also be formed by oxidation.
\ \
CH-NH-R + [O] ---> C=N-R + H[O]H
/ /
Oxidation is usually followed by hydrolysis,
unless the imine is stabilized as in a ring structure.
~~~ CONJUGATED IMINES AS REDOX ACTIVE COMPOUNDS ~~~
Structure Activity Relationships:
H H [O]
R--N--C==C--N--R' --->
H H
* H
R--N--C==C--N--R' <--->
H H
* H [O]
R--N==C--C--N--R' --->
H H
R--N==C--C==N--R'
H H
Note how the above is similar to the
dehydrogenation of enediols.
The above steps can be reversed
by adding back two hydrogen atoms.
IMPORTANT IMINE TYPE REDOX AGENTS IN BIOCHEMISTRY:
Pyridiniums Flavins Phenazines Biopterins
Porphins Pyrroloquinoline-quinones Folates
~~~ PYRIDINIUMS AS CARRIERS OF REDUCING EQUIVALENTS ~~~
H H H
[H] C C
/ \\ O H / \ O H
HC C--C--N HC C--C--N
|| | H || || H
HC CH HC CH
_ \ // \ /
e N+ N
R R
---------------------------------------------------------
THIOCTIC (LIPOIC) ACID:
Dihydrothioctic Acid (reduced):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
| | |
SH SH OH
Thioctic Acid (oxidized):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
\ / |
S---S OH
---------------------------------------------------------
ANALOGUES OF CYSTEINE:
---------------------------------------------------------
SERINE: COOH
\ H oxygen as in alcohol
HC--C--OH
/ H ^
NH2
---------------------------------------------------------
CYSTEINE: COOH
\ H sulfur as in thiol
HC--C--SH
/ H ^
NH2
---------------------------------------------------------
SELENOCYSTEINE: COOH
\ H selenium as in selenol
HC--C--SeH
/ H ^
NH2
---------------------------------------------------------
GLUTATHIONE (GSH) :
(gamma-glutamylcysteinylglycine)
COOH O O O
\ H H || H || H ||
C--C--C--C--N--C--C--N--C--C
/ H H H | H H \
NH2 HCH OH
|
SH
GLUTATHIYL RADICAL (GS*) :
[tripeptide]--S*
GLUTATHIONE DISULFIDE (GSSG) :
[tripeptide]--S--S--[tripeptide]
~~~ PROTEIN THIOLS ~~~
These include redox active cysteine residues
in their amino acid sequences.
They function as hydrogen carriers, physiologic
triggers, redox buffers, and oxidoreductases.
Examples: - Thioredoxin
- Glutaredoxin
- Protein Disulfide Isomerase
- Ref-1
- Transcription Factors
- Growth Factors
- Metallothionein
---------------------------------------------------------
PROTEIN THIOLS:
(Note reduced condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| | |
SH SH SH
(Note oxidized condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| \ /
S--S--R S--S
---------------------------------------------------------
~~~ MECHANISM OF OXIDO-REDUCTASES ~~~
NOTE: These use the thiol / disulfide half reaction
as part of the reductant transfer mechanism.
1) Red-H2 + Enz-SS ---> Red + Enz-(SH)2
2) Enz-(SH)2 + Ox ---> Ox-H2 + Enz-SS
- cys - - cys - - cys -
| | |
H S SH S H
[Red] | [Ox] ---> [Red] [Ox] ---> [Red] | [Ox]
H S SH S H
| | |
- cys - - cys - - cys -
~~~ MECHANISM OF INACTIVATION BY MERCURY II ~~~
Enz-(SH)2 + Hg++ ---> Enz-SHgS + 2H+
- cys - - cys - | - cys -
| | | |
SH S | H S
+ Hg++ ---> Hg + 2H+ | [Red] Hg [Ox]
SH S | H S
| | | |
- cys - - cys - | - cys -
~~~ ENOLS MECHANISM OF DEHYDROGENATION ~~~
Enols are composed of one hydroxyl group (HO)
attached to the vinylic carbon of an olefin.
OH
|
-C==C-
When oxidized by the abstraction of the
hydroxyl hydrogen, an alkoxyl radical is produced.
It is stabilized by two point resonance
with the conjugated vinyl group.
O* O
| <---> | *
-C==C- -C--C-
If this structure is further conjugated,
then fairly stable radicals can be produced.
~~~ UNPROTECTED PHENOXYL RADICAL ~~~
O* O O O
| || || ||
C C C C
// \ / \ / \ / \
HC CH HC* CH HC CH HC *CH
| || | || || || || |
HC CH HC CH HC CH HC CH
\\ / \\ / \ * / \ //
C C C C
H H H H
Phenoxyl radicals are stabilized well by 4 point resonance.
However, they are subject to addition reactions at each
of the 4 exposed radical sites shown: O, C#2, C#4, C#6.
~~~ PROTECTED PHENOXYL RADICALS ~~~
NOTE: The attached groups protect the four sites
of resonance against addition reactions.
-------------------------------------------------------
butylatedhydroxytoluene HC--C--C(CH3)3
RO* + BHT-OH --> // \\
ROH + BHT-O* CH3--C C--O*
\ /
HC==C--C(CH3)3
-------------------------------------------------------
alpha-tocopherol CH3 CH3--C--C--CH3
RO* + E-OH --> \ // \\
ROH + E-O* [turpene]--C---O--C C--O*
| \ /
CH2-CH2--C==C--CH3
-------------------------------------------------------
ubiquinol H3CO--C--C--OCH3
RO* + CoQ-OH --> // \\
ROH + CoQ-O* HO--C C--O*
\ /
[turpene]--C==C--CH3
-------------------------------------------------------
~~~ ENEDIOLS MECHANISM OF DEHYDROGENATION ~~~
Enediols are subject to facile one and two step
hydrogen abstractions.
STEP 1: OH OH O* OH O OH
| | + [O] ---> | | <---> || |
--C==C-- --C==C-- -C--C--
*
STEP 2: O OH O O* O O
|| | + [O] ---> || | ---> || ||
--C--C-- --C--C-- --C--C--
* *
The first abstraction is more difficult, because the
strong covalent oxyhydrogen sigma bond must be broken
to generate an alkoxyl radical.
However the opportunity for resonance stabilization
offerred by the conjugated vinyl group energetically
facilitates the abstraction.
Resonance towards the second hydroxyl group facilitates
release of the second hydrogen atom, because all
electrons of the final product can be paired.
This is an example of free radical quenching by oxidation.
~~~ ENEDIOL EXAMPLES ~~~
-------------------------------------------------
ASCORBIC ACID HO OH
\ /
H C==C
H O / \
HC--C--CH C==O
O H \ /
H O
-------------------------------------------------
CATACHOLS/ HO OH
ORTHOHYDROQUINOLS \ /
C==C
/ \
R--C C--R
\\ //
R--C--C--R
~~~ PARAHYDROQUINOL MECHANISM OF DEHYDROGENATION ~~~
Parahydroquinol (QH2):
HC==CH HC==CH
/ \ / \
HO-C C-OH + [O] ---> *O-C C-OH + *[O]H
\\ // \\ //
HC--CH HC--CH
Semiquinone (*QH):
HC==CH <---> HC==CH <---> HC==CH <---> HC--CH
/ \ / \ / \ /* \\
*O-C C-OH O=C C-OH O=C *C-OH O=C C-OH
\\ // \* // \ / \ /
HC--CH HC--CH HC==CH HC==CH
Paraquinone (Q):
HC==CH HC==CH
/ \ / \
O=C *C-OH + [O] ---> O=C C=O + *[O]H
\ / \ /
HC==CH HC==CH
~~~ IMINES ~~~
Imines (also known as Schiff's bases) are compounds
containing the carbon to nitrogen double bond.
C==N
They can be produced by the reaction of a carbonyl
group and a primary amino group at slightly acid pH.
\ H \ /OH \
C=O + N-R <---> C <---> C=N-R + HOH
/ H / \N-R /
H
As indicated by the arrows,
this reaction is usually reversible.
Imines can also be formed by oxidation.
\ \
CH-NH-R + [O] ---> C=N-R + H[O]H
/ /
Oxidation is usually followed by hydrolysis,
unless the imine is stabilized as in a ring structure.
~~~ CONJUGATED IMINES AS REDOX ACTIVE COMPOUNDS ~~~
Structure Activity Relationships:
H H [O]
R--N--C==C--N--R' --->
H H
* H
R--N--C==C--N--R' <--->
H H
* H [O]
R--N==C--C--N--R' --->
H H
R--N==C--C==N--R'
H H
Note how the above is similar to the
dehydrogenation of enediols.
The above steps can be reversed
by adding back two hydrogen atoms.
IMPORTANT IMINE TYPE REDOX AGENTS IN BIOCHEMISTRY:
Pyridiniums Flavins Phenazines Biopterins
Porphins Pyrroloquinoline-quinones Folates
~~~ PYRIDINIUMS AS CARRIERS OF REDUCING EQUIVALENTS ~~~
H H H
[H] C C
/ \\ O H / \ O H
HC C--C--N HC C--C--N
|| | H || || H
HC CH HC CH
_ \ // \ /
e N+ N
R R
HOME~~~PREV~~~NEXT
 Thiols are readily oxidized by a variety of oxidants
to produce the relatively stable thiyl radical (RS*).
RSH + [O] ---> RS* + H[O]*
Thiols are readily oxidized by a variety of oxidants
to produce the relatively stable thiyl radical (RS*).
RSH + [O] ---> RS* + H[O]*
 Thiyl radicals readily couple to produce disulfides (RSSR').
RS* + R'S* ---> RSSR'
Thiyl radicals readily couple to produce disulfides (RSSR').
RS* + R'S* ---> RSSR'
 These actions make thiols such as glutathione,
thioredoxin, and other peptides containing the
amino acid cysteine handy carriers of hydrogen atoms.
~~~ THIOL OXIDATION BY OXYRADICALS ~~~
Hydroxyl Radical:
HO* + RSH ---> HOH + RS*
Hydroperoxyl Radical:
HOO* + RSH ---> HOOH + RS*
Alkoxyl Radical:
RO* + RSH ---> ROH + RS*
Alkylperoxyl Radical:
ROO* + RSH ---> ROOH + RS*
~~~ THIOL OXIDATION BY TRANSITION METALS ~~~
RSH + Fe+++ ---> RS* + Fe++ + H+
~~~ THIOL OXIDATION BY QUINONES ~~~
RSH + Q ---> RS* + HQ*
~~~ THIOLS AS WEAK ACIDS ~~~
RSH + B- ---> RS- + HB
- Thiols can deprotonate at pH = 8.
- This produces the "thiolate anion" (RS-).
- Thiolate can function as a nucleophile.
- Thiolate is more sensitive to oxidation
than the corresponding thiol was.
~~~ THIOL ALDEHYDE ADDITIONS ~~~
O OH
R-S-H + C-R' <---> R-S-C-R'
H H
Thiols and aldehydes react by nucleophilic
addition to produce "thiohemiacetals".
By this mechanism, many enzymes having
thiol(s) as part of the active center are
reversibly inhibited by aldehydes.
Also by this mechanism, certain aldehydes
are detoxified by thiols.
~~~ THIOL / DISULFIDE EXCHANGE REACTIONS ~~~
Such reactions are catalized by thiolate anion and are
therefore facilitated by mildly alkaline conditions.
Thiols acting as nucleophiles can undergo
exchanges with disulfides:
RSH + R'SSR" ---> R'SSR + HSR"
Hydrogen sulfide similarly adds to disulfides
displacing a thiol:
HSH + R'SSR" ---> R'SSH + HSR"
Disulfides can exchange bound thyil groups:
RSSR' + R"SSR''' ---> RSSR''' + R"SSR'
~~~ THIOL OXIDATION TO DISULFIDE
BY NON-FREE-RADICAL MECHANISMS ~~~
RSH + R'SH + [O] ---> RSSR' + H[O]H
Examples below show the ubiquitous thiol glutathione
(GSH) being oxidized by:
sulfur: S8 + 16 GSH ---> 8 H2S + 8 GSSG
disulfide: RSSR + 2 GSH ---> 2 RSH + GSSG
sulfoxide: RSOR + 2 GSH ---> R-S-R + GSSG + H2O
disulfide monoxide:
RSSOR + 4 GSH ---> 2 RSH + 2 GSSG + H2O
diamide: D + 2 GSH ---> DH2 + GSSG
enzyme: Enz + 2 GSH ---> EnzH2 + GSSG
~~~ DISULFIDE-MONOXIDE AND THIOL REACTIONS ~~~
Disulfide-monoxides (RSOSR') first exchange
with thiols and then oxidize them.
RSOSR' + R"SH ---> RSOH + R'SSR"
RSOH + R"SH ---> HOH + RSSR"
The end result is two oxidized disulfides,
capable of absorbing four hydrogen atoms.
Allicin is a disulfide monoxide, a mild
oxidant, and an antibacterial agent.
H H O H H
C==C--C--S--S--C--C==C
H H H H H H
~~~ THIOL OXIDATION BY DIAMIDE ~~~
R'SH + R"SH + D ---> R'SSR" + DH2
- Diamide is a specific oxidant for thiols.
- It acts most rapidly upon low molecular
weight thiols such as glutathione.
- It works by a combined nucleophilic addition
& displacement mechanism, which cleanly
converts thiols to disulfides.
- It penetrates all compartments rapidly
and is effective both in vivo & in vitro.
- These features make diamide a useful probe
to study thiol to disulfide conversions.
------------------------------------------------
H3C O O CH3
\ || || /
N---C---N===N---C---N
/ \
H3C CH3
---------------------------------------------
R'--SH R'--S
---> \ H
--N==N-- --N--N--
---------------------------------------------
HS--R" R'--S--S--R"
R'--S
\ H ---> H H
--N--N-- --N--N--
---------------------------------------------
~~~ THIOLS AS CARRIERS OF ACYL GROUPS ~~~
R--S--C--R'
||
O
~~~ BIOLOGICAL THIOLS ~~~
NOTE: These naturally occuring thiols participate
in numerous physiologic functions and are readily
and reversibly oxidizable to disulfides.
"Co-Enzyme A" "Thioctic (Lipoic) Acid"
"Cysteine" "Glutathione" "Protein Thiols"
---------------------------------------------------------
CO-ENZYME A:
beta-amino-ethane-thiol 1'adenine
H H H /
HS--C--C--N--pantethine--5'pyrophosphate--ribose
H H \
3'phosphate
These actions make thiols such as glutathione,
thioredoxin, and other peptides containing the
amino acid cysteine handy carriers of hydrogen atoms.
~~~ THIOL OXIDATION BY OXYRADICALS ~~~
Hydroxyl Radical:
HO* + RSH ---> HOH + RS*
Hydroperoxyl Radical:
HOO* + RSH ---> HOOH + RS*
Alkoxyl Radical:
RO* + RSH ---> ROH + RS*
Alkylperoxyl Radical:
ROO* + RSH ---> ROOH + RS*
~~~ THIOL OXIDATION BY TRANSITION METALS ~~~
RSH + Fe+++ ---> RS* + Fe++ + H+
~~~ THIOL OXIDATION BY QUINONES ~~~
RSH + Q ---> RS* + HQ*
~~~ THIOLS AS WEAK ACIDS ~~~
RSH + B- ---> RS- + HB
- Thiols can deprotonate at pH = 8.
- This produces the "thiolate anion" (RS-).
- Thiolate can function as a nucleophile.
- Thiolate is more sensitive to oxidation
than the corresponding thiol was.
~~~ THIOL ALDEHYDE ADDITIONS ~~~
O OH
R-S-H + C-R' <---> R-S-C-R'
H H
Thiols and aldehydes react by nucleophilic
addition to produce "thiohemiacetals".
By this mechanism, many enzymes having
thiol(s) as part of the active center are
reversibly inhibited by aldehydes.
Also by this mechanism, certain aldehydes
are detoxified by thiols.
~~~ THIOL / DISULFIDE EXCHANGE REACTIONS ~~~
Such reactions are catalized by thiolate anion and are
therefore facilitated by mildly alkaline conditions.
Thiols acting as nucleophiles can undergo
exchanges with disulfides:
RSH + R'SSR" ---> R'SSR + HSR"
Hydrogen sulfide similarly adds to disulfides
displacing a thiol:
HSH + R'SSR" ---> R'SSH + HSR"
Disulfides can exchange bound thyil groups:
RSSR' + R"SSR''' ---> RSSR''' + R"SSR'
~~~ THIOL OXIDATION TO DISULFIDE
BY NON-FREE-RADICAL MECHANISMS ~~~
RSH + R'SH + [O] ---> RSSR' + H[O]H
Examples below show the ubiquitous thiol glutathione
(GSH) being oxidized by:
sulfur: S8 + 16 GSH ---> 8 H2S + 8 GSSG
disulfide: RSSR + 2 GSH ---> 2 RSH + GSSG
sulfoxide: RSOR + 2 GSH ---> R-S-R + GSSG + H2O
disulfide monoxide:
RSSOR + 4 GSH ---> 2 RSH + 2 GSSG + H2O
diamide: D + 2 GSH ---> DH2 + GSSG
enzyme: Enz + 2 GSH ---> EnzH2 + GSSG
~~~ DISULFIDE-MONOXIDE AND THIOL REACTIONS ~~~
Disulfide-monoxides (RSOSR') first exchange
with thiols and then oxidize them.
RSOSR' + R"SH ---> RSOH + R'SSR"
RSOH + R"SH ---> HOH + RSSR"
The end result is two oxidized disulfides,
capable of absorbing four hydrogen atoms.
Allicin is a disulfide monoxide, a mild
oxidant, and an antibacterial agent.
H H O H H
C==C--C--S--S--C--C==C
H H H H H H
~~~ THIOL OXIDATION BY DIAMIDE ~~~
R'SH + R"SH + D ---> R'SSR" + DH2
- Diamide is a specific oxidant for thiols.
- It acts most rapidly upon low molecular
weight thiols such as glutathione.
- It works by a combined nucleophilic addition
& displacement mechanism, which cleanly
converts thiols to disulfides.
- It penetrates all compartments rapidly
and is effective both in vivo & in vitro.
- These features make diamide a useful probe
to study thiol to disulfide conversions.
------------------------------------------------
H3C O O CH3
\ || || /
N---C---N===N---C---N
/ \
H3C CH3
---------------------------------------------
R'--SH R'--S
---> \ H
--N==N-- --N--N--
---------------------------------------------
HS--R" R'--S--S--R"
R'--S
\ H ---> H H
--N--N-- --N--N--
---------------------------------------------
~~~ THIOLS AS CARRIERS OF ACYL GROUPS ~~~
R--S--C--R'
||
O
~~~ BIOLOGICAL THIOLS ~~~
NOTE: These naturally occuring thiols participate
in numerous physiologic functions and are readily
and reversibly oxidizable to disulfides.
"Co-Enzyme A" "Thioctic (Lipoic) Acid"
"Cysteine" "Glutathione" "Protein Thiols"
---------------------------------------------------------
CO-ENZYME A:
beta-amino-ethane-thiol 1'adenine
H H H /
HS--C--C--N--pantethine--5'pyrophosphate--ribose
H H \
3'phosphate
 ---------------------------------------------------------
THIOCTIC (LIPOIC) ACID:
Dihydrothioctic Acid (reduced):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
| | |
SH SH OH
Thioctic Acid (oxidized):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
\ / |
S---S OH
---------------------------------------------------------
ANALOGUES OF CYSTEINE:
---------------------------------------------------------
SERINE: COOH
\ H oxygen as in alcohol
HC--C--OH
/ H ^
NH2
---------------------------------------------------------
CYSTEINE: COOH
\ H sulfur as in thiol
HC--C--SH
/ H ^
NH2
---------------------------------------------------------
SELENOCYSTEINE: COOH
\ H selenium as in selenol
HC--C--SeH
/ H ^
NH2
---------------------------------------------------------
GLUTATHIONE (GSH) :
(gamma-glutamylcysteinylglycine)
COOH O O O
\ H H || H || H ||
C--C--C--C--N--C--C--N--C--C
/ H H H | H H \
NH2 HCH OH
|
SH
GLUTATHIYL RADICAL (GS*) :
[tripeptide]--S*
GLUTATHIONE DISULFIDE (GSSG) :
[tripeptide]--S--S--[tripeptide]
~~~ PROTEIN THIOLS ~~~
These include redox active cysteine residues
in their amino acid sequences.
They function as hydrogen carriers, physiologic
triggers, redox buffers, and oxidoreductases.
Examples: - Thioredoxin
- Glutaredoxin
- Protein Disulfide Isomerase
- Ref-1
- Transcription Factors
- Growth Factors
- Metallothionein
---------------------------------------------------------
PROTEIN THIOLS:
(Note reduced condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| | |
SH SH SH
(Note oxidized condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| \ /
S--S--R S--S
---------------------------------------------------------
~~~ MECHANISM OF OXIDO-REDUCTASES ~~~
NOTE: These use the thiol / disulfide half reaction
as part of the reductant transfer mechanism.
1) Red-H2 + Enz-SS ---> Red + Enz-(SH)2
2) Enz-(SH)2 + Ox ---> Ox-H2 + Enz-SS
- cys - - cys - - cys -
| | |
H S SH S H
[Red] | [Ox] ---> [Red] [Ox] ---> [Red] | [Ox]
H S SH S H
| | |
- cys - - cys - - cys -
~~~ MECHANISM OF INACTIVATION BY MERCURY II ~~~
Enz-(SH)2 + Hg++ ---> Enz-SHgS + 2H+
- cys - - cys - | - cys -
| | | |
SH S | H S
+ Hg++ ---> Hg + 2H+ | [Red] Hg [Ox]
SH S | H S
| | | |
- cys - - cys - | - cys -
~~~ ENOLS MECHANISM OF DEHYDROGENATION ~~~
Enols are composed of one hydroxyl group (HO)
attached to the vinylic carbon of an olefin.
OH
|
-C==C-
When oxidized by the abstraction of the
hydroxyl hydrogen, an alkoxyl radical is produced.
It is stabilized by two point resonance
with the conjugated vinyl group.
O* O
| <---> | *
-C==C- -C--C-
If this structure is further conjugated,
then fairly stable radicals can be produced.
~~~ UNPROTECTED PHENOXYL RADICAL ~~~
O* O O O
| || || ||
C C C C
// \ / \ / \ / \
HC CH HC* CH HC CH HC *CH
| || | || || || || |
HC CH HC CH HC CH HC CH
\\ / \\ / \ * / \ //
C C C C
H H H H
Phenoxyl radicals are stabilized well by 4 point resonance.
However, they are subject to addition reactions at each
of the 4 exposed radical sites shown: O, C#2, C#4, C#6.
~~~ PROTECTED PHENOXYL RADICALS ~~~
NOTE: The attached groups protect the four sites
of resonance against addition reactions.
-------------------------------------------------------
butylatedhydroxytoluene HC--C--C(CH3)3
RO* + BHT-OH --> // \\
ROH + BHT-O* CH3--C C--O*
\ /
HC==C--C(CH3)3
-------------------------------------------------------
alpha-tocopherol CH3 CH3--C--C--CH3
RO* + E-OH --> \ // \\
ROH + E-O* [turpene]--C---O--C C--O*
| \ /
CH2-CH2--C==C--CH3
-------------------------------------------------------
ubiquinol H3CO--C--C--OCH3
RO* + CoQ-OH --> // \\
ROH + CoQ-O* HO--C C--O*
\ /
[turpene]--C==C--CH3
-------------------------------------------------------
~~~ ENEDIOLS MECHANISM OF DEHYDROGENATION ~~~
Enediols are subject to facile one and two step
hydrogen abstractions.
STEP 1: OH OH O* OH O OH
| | + [O] ---> | | <---> || |
--C==C-- --C==C-- -C--C--
*
STEP 2: O OH O O* O O
|| | + [O] ---> || | ---> || ||
--C--C-- --C--C-- --C--C--
* *
The first abstraction is more difficult, because the
strong covalent oxyhydrogen sigma bond must be broken
to generate an alkoxyl radical.
However the opportunity for resonance stabilization
offerred by the conjugated vinyl group energetically
facilitates the abstraction.
Resonance towards the second hydroxyl group facilitates
release of the second hydrogen atom, because all
electrons of the final product can be paired.
This is an example of free radical quenching by oxidation.
~~~ ENEDIOL EXAMPLES ~~~
-------------------------------------------------
ASCORBIC ACID HO OH
\ /
H C==C
H O / \
HC--C--CH C==O
O H \ /
H O
-------------------------------------------------
CATACHOLS/ HO OH
ORTHOHYDROQUINOLS \ /
C==C
/ \
R--C C--R
\\ //
R--C--C--R
~~~ PARAHYDROQUINOL MECHANISM OF DEHYDROGENATION ~~~
Parahydroquinol (QH2):
HC==CH HC==CH
/ \ / \
HO-C C-OH + [O] ---> *O-C C-OH + *[O]H
\\ // \\ //
HC--CH HC--CH
Semiquinone (*QH):
HC==CH <---> HC==CH <---> HC==CH <---> HC--CH
/ \ / \ / \ /* \\
*O-C C-OH O=C C-OH O=C *C-OH O=C C-OH
\\ // \* // \ / \ /
HC--CH HC--CH HC==CH HC==CH
Paraquinone (Q):
HC==CH HC==CH
/ \ / \
O=C *C-OH + [O] ---> O=C C=O + *[O]H
\ / \ /
HC==CH HC==CH
~~~ IMINES ~~~
Imines (also known as Schiff's bases) are compounds
containing the carbon to nitrogen double bond.
C==N
They can be produced by the reaction of a carbonyl
group and a primary amino group at slightly acid pH.
\ H \ /OH \
C=O + N-R <---> C <---> C=N-R + HOH
/ H / \N-R /
H
As indicated by the arrows,
this reaction is usually reversible.
Imines can also be formed by oxidation.
\ \
CH-NH-R + [O] ---> C=N-R + H[O]H
/ /
Oxidation is usually followed by hydrolysis,
unless the imine is stabilized as in a ring structure.
~~~ CONJUGATED IMINES AS REDOX ACTIVE COMPOUNDS ~~~
Structure Activity Relationships:
H H [O]
R--N--C==C--N--R' --->
H H
* H
R--N--C==C--N--R' <--->
H H
* H [O]
R--N==C--C--N--R' --->
H H
R--N==C--C==N--R'
H H
Note how the above is similar to the
dehydrogenation of enediols.
The above steps can be reversed
by adding back two hydrogen atoms.
IMPORTANT IMINE TYPE REDOX AGENTS IN BIOCHEMISTRY:
Pyridiniums Flavins Phenazines Biopterins
Porphins Pyrroloquinoline-quinones Folates
~~~ PYRIDINIUMS AS CARRIERS OF REDUCING EQUIVALENTS ~~~
H H H
[H] C C
/ \\ O H / \ O H
HC C--C--N HC C--C--N
|| | H || || H
HC CH HC CH
_ \ // \ /
e N+ N
R R
---------------------------------------------------------
THIOCTIC (LIPOIC) ACID:
Dihydrothioctic Acid (reduced):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
| | |
SH SH OH
Thioctic Acid (oxidized):
CH2-CH2-CH-CH2-CH2-CH2-CH2-C=O
\ / |
S---S OH
---------------------------------------------------------
ANALOGUES OF CYSTEINE:
---------------------------------------------------------
SERINE: COOH
\ H oxygen as in alcohol
HC--C--OH
/ H ^
NH2
---------------------------------------------------------
CYSTEINE: COOH
\ H sulfur as in thiol
HC--C--SH
/ H ^
NH2
---------------------------------------------------------
SELENOCYSTEINE: COOH
\ H selenium as in selenol
HC--C--SeH
/ H ^
NH2
---------------------------------------------------------
GLUTATHIONE (GSH) :
(gamma-glutamylcysteinylglycine)
COOH O O O
\ H H || H || H ||
C--C--C--C--N--C--C--N--C--C
/ H H H | H H \
NH2 HCH OH
|
SH
GLUTATHIYL RADICAL (GS*) :
[tripeptide]--S*
GLUTATHIONE DISULFIDE (GSSG) :
[tripeptide]--S--S--[tripeptide]
~~~ PROTEIN THIOLS ~~~
These include redox active cysteine residues
in their amino acid sequences.
They function as hydrogen carriers, physiologic
triggers, redox buffers, and oxidoreductases.
Examples: - Thioredoxin
- Glutaredoxin
- Protein Disulfide Isomerase
- Ref-1
- Transcription Factors
- Growth Factors
- Metallothionein
---------------------------------------------------------
PROTEIN THIOLS:
(Note reduced condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| | |
SH SH SH
(Note oxidized condition.)
H H O H H O H H O H H O H H O
HN--C--C--N--C--C--N--C--C--N--C--C--N--C--COH
| | | | |
HCH X X HCH HCH
| \ /
S--S--R S--S
---------------------------------------------------------
~~~ MECHANISM OF OXIDO-REDUCTASES ~~~
NOTE: These use the thiol / disulfide half reaction
as part of the reductant transfer mechanism.
1) Red-H2 + Enz-SS ---> Red + Enz-(SH)2
2) Enz-(SH)2 + Ox ---> Ox-H2 + Enz-SS
- cys - - cys - - cys -
| | |
H S SH S H
[Red] | [Ox] ---> [Red] [Ox] ---> [Red] | [Ox]
H S SH S H
| | |
- cys - - cys - - cys -
~~~ MECHANISM OF INACTIVATION BY MERCURY II ~~~
Enz-(SH)2 + Hg++ ---> Enz-SHgS + 2H+
- cys - - cys - | - cys -
| | | |
SH S | H S
+ Hg++ ---> Hg + 2H+ | [Red] Hg [Ox]
SH S | H S
| | | |
- cys - - cys - | - cys -
~~~ ENOLS MECHANISM OF DEHYDROGENATION ~~~
Enols are composed of one hydroxyl group (HO)
attached to the vinylic carbon of an olefin.
OH
|
-C==C-
When oxidized by the abstraction of the
hydroxyl hydrogen, an alkoxyl radical is produced.
It is stabilized by two point resonance
with the conjugated vinyl group.
O* O
| <---> | *
-C==C- -C--C-
If this structure is further conjugated,
then fairly stable radicals can be produced.
~~~ UNPROTECTED PHENOXYL RADICAL ~~~
O* O O O
| || || ||
C C C C
// \ / \ / \ / \
HC CH HC* CH HC CH HC *CH
| || | || || || || |
HC CH HC CH HC CH HC CH
\\ / \\ / \ * / \ //
C C C C
H H H H
Phenoxyl radicals are stabilized well by 4 point resonance.
However, they are subject to addition reactions at each
of the 4 exposed radical sites shown: O, C#2, C#4, C#6.
~~~ PROTECTED PHENOXYL RADICALS ~~~
NOTE: The attached groups protect the four sites
of resonance against addition reactions.
-------------------------------------------------------
butylatedhydroxytoluene HC--C--C(CH3)3
RO* + BHT-OH --> // \\
ROH + BHT-O* CH3--C C--O*
\ /
HC==C--C(CH3)3
-------------------------------------------------------
alpha-tocopherol CH3 CH3--C--C--CH3
RO* + E-OH --> \ // \\
ROH + E-O* [turpene]--C---O--C C--O*
| \ /
CH2-CH2--C==C--CH3
-------------------------------------------------------
ubiquinol H3CO--C--C--OCH3
RO* + CoQ-OH --> // \\
ROH + CoQ-O* HO--C C--O*
\ /
[turpene]--C==C--CH3
-------------------------------------------------------
~~~ ENEDIOLS MECHANISM OF DEHYDROGENATION ~~~
Enediols are subject to facile one and two step
hydrogen abstractions.
STEP 1: OH OH O* OH O OH
| | + [O] ---> | | <---> || |
--C==C-- --C==C-- -C--C--
*
STEP 2: O OH O O* O O
|| | + [O] ---> || | ---> || ||
--C--C-- --C--C-- --C--C--
* *
The first abstraction is more difficult, because the
strong covalent oxyhydrogen sigma bond must be broken
to generate an alkoxyl radical.
However the opportunity for resonance stabilization
offerred by the conjugated vinyl group energetically
facilitates the abstraction.
Resonance towards the second hydroxyl group facilitates
release of the second hydrogen atom, because all
electrons of the final product can be paired.
This is an example of free radical quenching by oxidation.
~~~ ENEDIOL EXAMPLES ~~~
-------------------------------------------------
ASCORBIC ACID HO OH
\ /
H C==C
H O / \
HC--C--CH C==O
O H \ /
H O
-------------------------------------------------
CATACHOLS/ HO OH
ORTHOHYDROQUINOLS \ /
C==C
/ \
R--C C--R
\\ //
R--C--C--R
~~~ PARAHYDROQUINOL MECHANISM OF DEHYDROGENATION ~~~
Parahydroquinol (QH2):
HC==CH HC==CH
/ \ / \
HO-C C-OH + [O] ---> *O-C C-OH + *[O]H
\\ // \\ //
HC--CH HC--CH
Semiquinone (*QH):
HC==CH <---> HC==CH <---> HC==CH <---> HC--CH
/ \ / \ / \ /* \\
*O-C C-OH O=C C-OH O=C *C-OH O=C C-OH
\\ // \* // \ / \ /
HC--CH HC--CH HC==CH HC==CH
Paraquinone (Q):
HC==CH HC==CH
/ \ / \
O=C *C-OH + [O] ---> O=C C=O + *[O]H
\ / \ /
HC==CH HC==CH
~~~ IMINES ~~~
Imines (also known as Schiff's bases) are compounds
containing the carbon to nitrogen double bond.
C==N
They can be produced by the reaction of a carbonyl
group and a primary amino group at slightly acid pH.
\ H \ /OH \
C=O + N-R <---> C <---> C=N-R + HOH
/ H / \N-R /
H
As indicated by the arrows,
this reaction is usually reversible.
Imines can also be formed by oxidation.
\ \
CH-NH-R + [O] ---> C=N-R + H[O]H
/ /
Oxidation is usually followed by hydrolysis,
unless the imine is stabilized as in a ring structure.
~~~ CONJUGATED IMINES AS REDOX ACTIVE COMPOUNDS ~~~
Structure Activity Relationships:
H H [O]
R--N--C==C--N--R' --->
H H
* H
R--N--C==C--N--R' <--->
H H
* H [O]
R--N==C--C--N--R' --->
H H
R--N==C--C==N--R'
H H
Note how the above is similar to the
dehydrogenation of enediols.
The above steps can be reversed
by adding back two hydrogen atoms.
IMPORTANT IMINE TYPE REDOX AGENTS IN BIOCHEMISTRY:
Pyridiniums Flavins Phenazines Biopterins
Porphins Pyrroloquinoline-quinones Folates
~~~ PYRIDINIUMS AS CARRIERS OF REDUCING EQUIVALENTS ~~~
H H H
[H] C C
/ \\ O H / \ O H
HC C--C--N HC C--C--N
|| | H || || H
HC CH HC CH
_ \ // \ /
e N+ N
R R